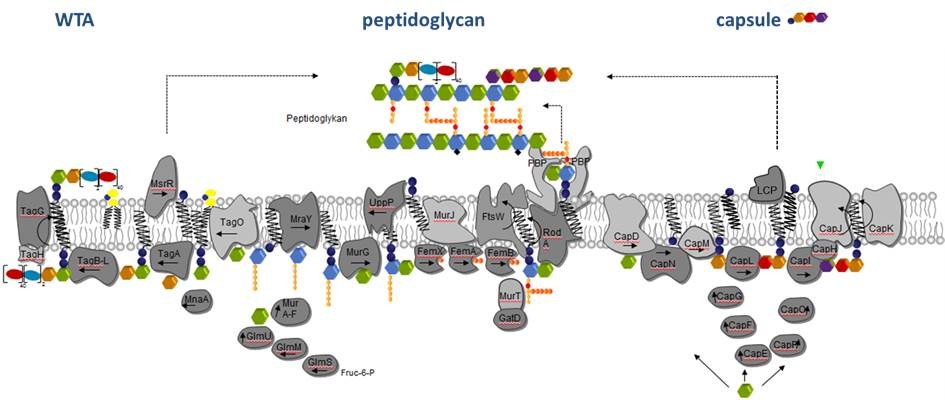
The Great Wall - cell envelope biosynthesis networks
The bacterial cell wall represents a crucial protective structure for bacteria and its integrity is of critical importance to cell viability. The cell wall structure is maintained by the combined activities of an intricate set of enzymes organized in multi-enzyme machineries spanning intra- and extracellular compartments. To ensure cell viability during growth and division, biosynthetic processes thus need to be tightly coordinated in time and space. The AG Schneider investigates the biosynthesis of the major cell wall components in Gram-positive bacteria – peptidoglycan (PG), wall teichoic acid (WTA) and capsule (CP).
Central to our studies is the synthesis of bactoprenol-coupled lipid intermediates, such as the PG precursor lipid II, which represents the cardinal cell wall building block. We are particularly interested in the membrane embedded biosynthesis reactions and the organization of the different cell envelope biosynthesis machineries.
Tool time – Antibiotics as tools to understand biological processes and cellular networks. “Reverse biochemistry” - dissecting cellular processes using inhibitors represents a highly successful strategy to understand biological processes.
MurT/GatD: Lipid II amidation-
It takes two to tango